Jan 30, 2025
Diana Driscoll
Optometrist
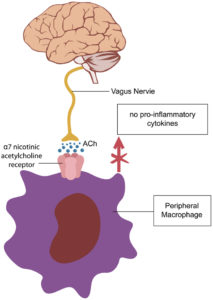
The recent surge in interest around the vagus nerve has led many to seek methods to stimulate their vagus nerve – everything from humming or splashing water on their face to using expensive vagus nerve stimulators. However, it's essential to realize that the vagus..
Jan 30, 2025
Diana Driscoll
Optometrist
The recent surge in interest around the vagus nerve has led many to seek methods to stimulate their vagus nerve – everything from humming or splashing water on their face to using expensive vagus nerve stimulators. However, it's essential to realize that the vagus..
Jan 30, 2025
Diana Driscoll
Optometrist
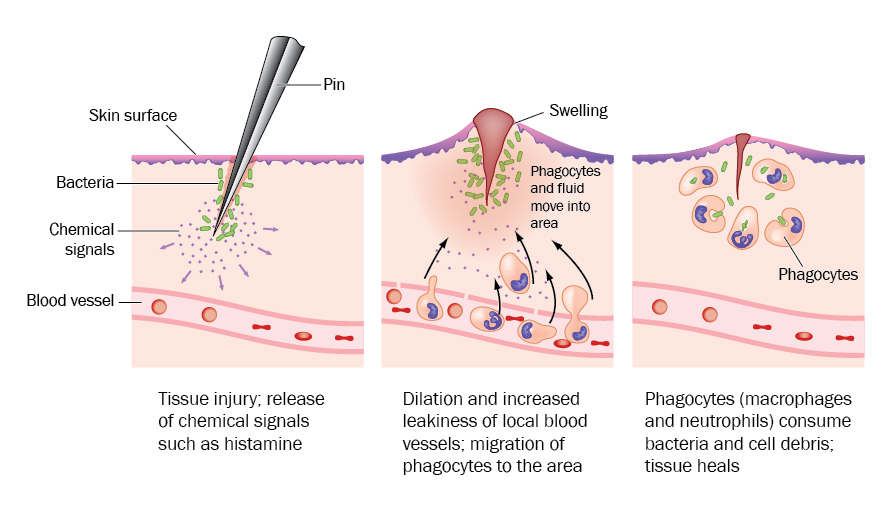
Acetylcholine is one of the body’s most important neurotransmitters. You can’t see it, you can’t measure it, but it is critical for your health. Acetylcholine is a neurotransmitter — a chemical messenger that allows your nerves to communicate.
Jan 30, 2025
Diana Driscoll
Optometrist
Acetylcholine is one of the body’s most important neurotransmitters. You can’t see it, you can’t measure it, but it is critical for your health. Acetylcholine is a neurotransmitter — a chemical messenger that allows your nerves to communicate.
Jan 30, 2025
Diana Driscoll
Optometrist
Patients suffering from Ehlers-Danlos syndrome (EDS) often experience a myriad of ocular symptoms. These conditions are addressed in my latest book, “Ehlers-Danlos Syndrome: Your Eyes and EDS Updated” and which conditions can be detrimental or which are merely...
Jan 30, 2025
Diana Driscoll
Optometrist
Patients suffering from Ehlers-Danlos syndrome (EDS) often experience a myriad of ocular symptoms. These conditions are addressed in my latest book, “Ehlers-Danlos Syndrome: Your Eyes and EDS Updated” and which conditions can be detrimental or which are merely...